|
|
Alexion is committed to the rare disease community
Please see full Prescribing
Information for
ULTOMIRIS®
(ravulizumab-cwvz)
including
Boxed WARNING regarding serious and life-threatening or fatal meningococcal
infections.
|
|
 |
SELECT IMPORTANT SAFETY INFORMATION
|
 |

|
 |
WARNING: SERIOUS MENINGOCOCCAL INFECTIONS
|
 |
 |
ULTOMIRIS, a complement inhibitor, increases the risk of serious infections caused
by Neisseria
meningitidis [see Warnings and Precautions (5.1)].
Life-threatening
and fatal
meningococcal
infections have occurred in patients treated with complement inhibitors. These
infections may
become rapidly life-threatening or fatal if not recognized and treated early.
|
 |
|

|
 |
Please see additional Important Safety Information, including full Boxed
WARNING,
below.
|
 |
|
|
gMG is a rare, debilitating, neuromuscular, heterogenous autoimmune
disorder characterized by fluctuating fatigable muscle weakness that can
impact activities of daily living2-4
|
|
Corticosteroids are the most commonly prescribed first-line immunosuppressive
therapy
(IST) for
gMG, but they may exacerbate or worsen certain comorbidities.5,6 Adult patients with
gMG
have a
high comorbidity burden associated with cardiovascular and endocrine
disorders.6,7
|
|
Baseline comorbidities reported in ≥15% of adult patients with gMG7,a
|
|
Despite the use of conventional therapies, some patients with gMG
are unable to tolerate multiple treatments and are vulnerable to
exacerbations and life-threatening crises8-10
|
|
ULTOMIRIS efficacy data from CHAMPION-MGc
|
|
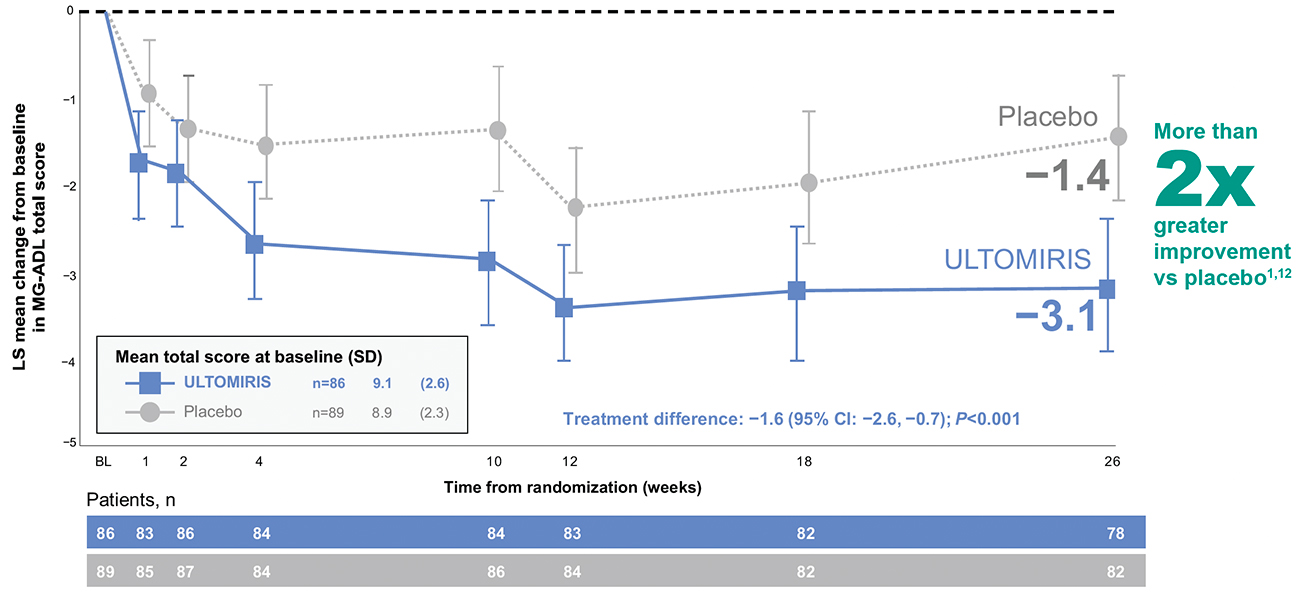
Among patients in the ULTOMIRIS arm, improvements in Myasthenia Gravis Activities of
Daily
Living (MG-ADL) total scores were observed within 1 week of treatment and were sustained
through Week 26 of treatment1,12,c
|
|
Primary endpoint: change from baseline in MG-ADL total score at 26 weeks1,12,d
|
|
ULTOMIRIS demonstrated efficacy vs placebo at Week 26 (-3.1 vs -1.4, respectively,
[P<0.001]).
|
|
|
CHAMPION-MG STUDY LIMITATIONS: Data shown are least-squares means and 95%
CIs, using a
mixed model for repeated measures; 95% CIs were not adjusted for multiplicity.
Time to response was part of the planned efficacy analysis, but the primary endpoint was at
Week 26.
Therefore, results should be interpreted with caution.
|
|
Time to response was part of the planned efficacy analysis, but the primary endpoint was at
Week 26.
Therefore, results should be interpreted with caution.
|

|
|
ULTOMIRIS safety profile
|
|
The most frequent adverse reactions occurring in ≥5% of patients in the ULTOMIRIS
treatment arm in CHAMPION-MG were diarrhea, abdominal pain, upper respiratory
tract infection, urinary tract infection, back pain, and dizziness.1
|
|
|
|
ULTOMIRIS post hoc analysis from CHAMPION-MG open-label extension (OLE)e
|
|
Reductions in corticosteroid daily use were observed in patients treated with
ULTOMIRIS
through Week 164 of the OLE period13,e
|
|
Exploratory endpoint: reduction in corticosteroid use13,14
|
|
By the last OLE assessment, an approximate 33% reduction in mean daily corticosteroid dose was
observed among patients with gMG taking corticosteroids at any point during the OLE (n=113).
|
|
Mean daily dose of corticosteroids in the OLE
|
|
CHAMPION-MG OLE STUDY LIMITATION: Results should be interpreted with caution
since the OLE
study was designed to evaluate safety and lacked a control group.
|
|
|
Weight-based dosing with once-every-8-week maintenance starting 2
weeks after initial loading dose1
|
|
Patients may require as few as 6 to 7 maintenance infusions per year.
|
|
|
Key: BL, baseline; C5, complement component 5;
CI, confidence interval; LS,
least squares; SD, standard deviation; US, United
States.
a. Data obtained from a US-based retrospective, observational cohort study
which utilized
Inovalon 100% Medicare Parts A and B
fee-for-service claims and 100% Part D Prescription Drug Event claims (from January 1, 2016,
to December 31, 2021) to characterize
comorbidity burden and steroid use in adult patients (≥18 years of age at index date
[January
1, 2017]) with gMG in the US (N=29,349).7
b. Data obtained from a US-based retrospective, observational cohort study
which utilized
patient medical and pharmacy claims data
(from January 1, 2006, to June 30, 2019) to quantify specific elements of disease burden
(incidence of crisis, exacerbations, and health
care resource utilization) in patients with inadequately controlled MG (reflected by use of
multiple ISTs and/or chronic IVIg) ≥18 years old
who had 1 or more claims that included diagnosis of MG with neurology or ophthalmology listed
as the provider specialty, at least 2 claims
with MG diagnosis, and ≥1 year of continuous enrollment before and after MG diagnosis.11
c.
ULTOMIRIS was evaluated in a phase 3,
randomized, double-blind, placebo-controlled, multicenter study assessing its safety and
efficacy in complement inhibitor-naïve adults with
gMG who were anti-AChR-Ab+. Patients received either ULTOMIRIS (n=86) or placebo (n=89) over a
26-week treatment period.1,12 d.
The
MG-ADL is a categorical scale that assesses the impact on daily function of 8 signs or
symptoms that are typically affected in gMG. Each
item is assessed on a 4-point scale where a score of 0 represents normal function and a score
of 3 represents loss of ability to perform
that function. The total score ranges from 0 to 24, with the higher scores indicating more
impairment.1
e. The CHAMPION-MG OLE
followed the 26-week randomized, double-blind, placebo-controlled core period of the
CHAMPION-MG trial, which evaluated the efficacy
and safety of ULTOMIRIS in adults with gMG who were anti-AChR-Ab+. In the OLE phase, all
participants transitioned to receive openlabel
ULTOMIRIS to assess the long-term safety, efficacy, and impact on patient-reported outcomes
over a period of up to 4 years.13
|

|

|
|
Alexion Access Navigator is a dedicated resource website for US
Healthcare Professionals and their offices that contains downloadable
access and reimbursement materials for ULTOMIRIS.
|
|
Online: alexionaccessnavigator.com
|
|
|
|
OneSource™ Offers Patient Support
|
|
IMPORTANT SAFETY INFORMATION & INDICATION
|
|
IMPORTANT SAFETY INFORMATION
|
| | | | | | |
